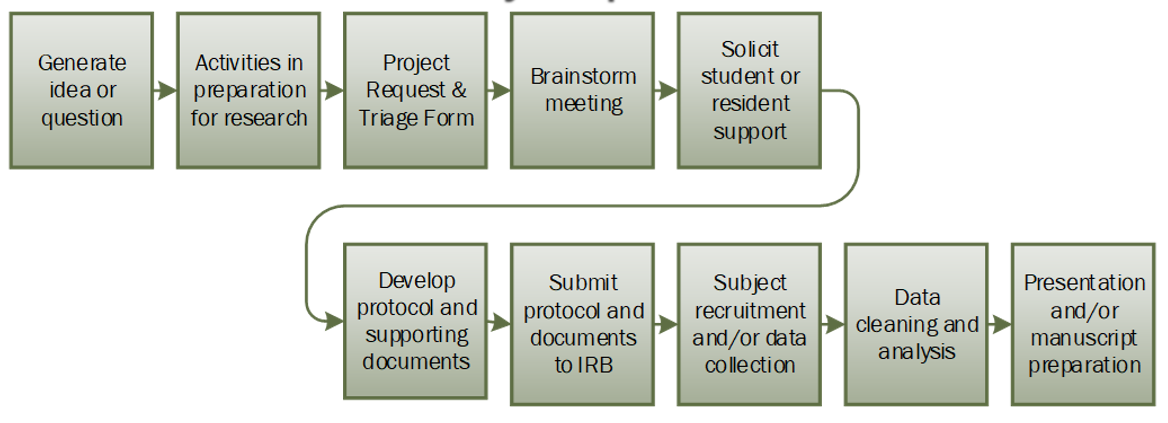
Investigator-initiated studies are projects conceived, organized, and implemented by WMed Faculty. To assist with investigator-initiated research, WMed has a number of research support services available. To set up your project and access these services, follow the guidance below. Please note, a Faculty Principal Investigator (PI) is required for student and resident project designs.
-
To begin your project, consider what you would like to research and what your hypothesis and outcomes could be. Keep the question obtainable, logical, and concise and keep data variables to the minimum necessary to answer your question.
PICOT-Population, Intervention, Comparisons, Primary outcome, Time:
- What is your target Population?
- Is there an Intervention?
- Will statistical analysis be adjusted for multiple Comparisons?
- What is your Primary outcome?
- Time – what is the duration of our data collection?
FINER- Feasibility, Interest, Novelty, Ethics, Relevance- Is it Feasible? Does it prioritize measureable outcomes or aims at achievable sample size?
- Is it Interesting? Does it attract attention or present a different prospective?
- Is it Novel? Does it provide new or different findings?
- Is it Ethical? Does it safeguard the principals of ethical research?
- Is it Relevant? Does it generate new knowledge or improve practice?
-
To determine if your idea has been previously researched, the WMed Medical Library is an excellent resource for advanced literature searches and guidance on research methodology.
A faculty mentor is required for all WMed projects. If you need assistance finding a mentor, please contact the Research Navigator.
The Data Manager for the WMed Virtual Data Warehouse can assist with the feasibility of your project with regards to the availability of electronic health record data and REDCap builds. Per HIPAA regulations (Privacy Rule), investigators can use private health information when assessing the feasibility of conducting a research project, developing a grant application, or identifying potential subjects. Contact the Data Manager at datawarehouse@wmed.edu to discuss potential options.
Learner Tips for Communicating with Your Mentor
Ask questions such as:
- What is your preferred method of contact?
- How long should I wait to follow up?
- What should I have prepared for our meetings?
- How do you like information presented?
- What is our project goal?
- What is a reasonable timeline?
Be prepared to let your faculty mentor know if you have any scheduling conflicts or unexpected struggles.If you run into mentoring challenges, please reach out to the Research navigator.
-
To start the protocol development process, please complete the Project Request & Triage Form. The form asks basic questions about what your project will entail including a summary of the project, where the data will be collected, and if you would like guidance with project development. Submission of the form prompts an alert to WMed’s research support services: the Research Navigator, Data Manager, Biostatisticians, Health Information Management, and IRB staff. The Research Navigator will contact you to confirm your project needs and to request dates and times you (and your team) are available for a brainstorming meeting. If you have a protocol draft available, it can be uploaded on the Project Request & Triage Form.
-
The project initiation meeting is an opportunity for you to summarize your project idea with the research support services team. At this meeting, you can request help with protocol development, feasibility, data collection and availability of data, power analysis, REDCap survey or data collection tools, and regulatory guidance. The members present at the meeting may vary depending on the information provided on the Project Request & Triage Form, and the PI must be available for the meeting. For the meeting, we ask that you have your research questions prepared and reviewed by your PI and plan to give a 5-10 minute synopsis of your project idea. The support services team members will come prepared with questions about the study design to ensure proper support is being provided. The principal investigator must be in attendance at this meeting.
The Research Navigator may set up a pre-meeting, if needed.
Tips to Prepare for the Meeting
- Discuss project with PI before the meeting
- Get clinical or educational approvals, as needed
- Prepare a draft protocol or, at minimum, the research questions you are planning to analyze
- Prepare to give a 5-10 minute overview of the project
If you have any questions about the meeting, please reach out to the Research Navigator. -
The WMed Research Opportunities portal allows for the solicitation of project help from medical students and residents. Please submit your research opportunity to have your project posted on the portal.
If you are a student or a resident looking to assist with a research or process improvement project, please visit the Research Opportunities portal page (Login Required). Opportunities are added as they become available, and an announcement will be posted in the Student Newsletter. If you are interested in a position, please apply in a timely manner. Postings go quickly. If you have any questions or would like to discuss opportunities, please reach out to the Research Navigator.

-
Protocol templates and descriptions are available through the HRPP/IRB website. Choose the template the most closely matches the project you are proposing.
The Research Support Team can assist with protocol development questions and needs. They will provide help in thinking through our project to develop the protocol and other documents, and define the study logistics. They will also help ensure your protocol contains all the appropriate pieces and supporting documentation (consent, survey language, data variables spreadsheet, etc.) required for IRB submission.
If you are working with the Data Manager and/or Biostatistician, they will assist you with the completion of the data management plan and statistical analysis plan.
Tips to Avoid Common Mistakes During Protocol Development
- Do a thorough literature review. Information can be used in the protocol, presentation and/or manuscript, and previous research can provide insight into replicating methods and avoiding pitfalls.
- Check the feasibility of your project: Is the participant population available? Do you have institutional approval? Is it process improvement or research project?
- What resources do you need to complete the project? Residents and medical students may be available to help.
- What is your timeline? EHR data requests take up to several months, depending on the source and current queue.
-
When the protocol is finalized, it can be submitted in iMedRIS. All study team members must be listed on the application and should have completed the appropriate CITI training. Ensure your application is consistent: Protocol, supporting documents, and the application should match (dates, number of participants, etc.). The project will be reviewed for IRB determination. Once reviewed, an outcome letter will be sent to the team.
-
After an IRB determination is granted, subjects should be recruited, and data collected as described in your study protocol. If modifications to the study design and procedures are needed, please contact the IRB at irb@wmed.edu to determine if your IRB application needs to be updated.
The preferred storage locations for data are in the Virtual Data Warehouse SharePoint workspace or REDCap. The Data Manager can assist with the set up and access to either (or both) location(s), depending on your study needs, and acts as the honest broker for data de-identification and coding.
The PI is ultimately responsible and should keep tabs on the study team. Routinely meet and review the protocol to ensure it is being followed and all variables are clearly defined and collected consistently.
-
As part of the Data Analytics Services Unit, the Data Manager and Biostatisticians can assist in the validation, cleanup, and analysis of your study data. They will work with you to confirm the data is being presented and analyzed as described via the objectives and outcomes listed in your study protocol. The statisticians can help develop analysis plans, sample size calculations, and power analysis; assist in writing the statistical analysis section of the protocol; and perform statistical analysis at the end of the study.
The Data Manager will track your data set to ensure it is only stored for the time frame determined by your protocol and IRB application. If the data set needs to be kept longer, or archived per NIH or journal request, the Data Manager can assist with updating this language and storing the data in a data repository.
-
The WMed library can assist with scholarly writing. The Data Manager can assist with methods. The biostatisticians can assist with statistical methods and results. Depending on the current workflow, the biostatisticians can also assist with tables and figures; during peak times, however, the biostatisticians may send output for study teams to format.
It is recommended to use the info in your protocol to create your manuscript/presentation. Have one person review and edit the entire final manuscript to ensure the language is consistent throughout.

