
Momoko Yoshimoto, MD, PhD, obtained her medical degree in Japan and completed a comprehensive five-year residency/fellowship in general Pediatrics and Pediatric Hematology-Oncology followed by one-year attending Pediatric Hematology-Oncologist. Dr. Yoshimoto involved dedicated care for pediatric leukemic patients through chemotherapy and stem cell transplantation, fueling her passion for advancing hematology research. This pursuit led her to earn a Ph.D. from Kyoto University Graduate School of Medicine in 2003.
In 2005, Dr. Yoshimoto joined Dr. Mervin Yoder's lab at Indiana University School of Medicine as a postdoctoral researcher. Her groundbreaking work there unveiled the solid evidence of lymphoid potential in the extra-embryonic yolk sac of mouse embryos. She was appointed Research Assistant Professor in 2011, securing R56 & R01 grants in 2015 and 2016. Her trajectory led her to be recruited as an Associate Professor at the Center for Stem Cell and Regenerative Medicine at McGovern Medical School, University of Texas Health Science Center at Houston, where she established her laboratory.
In September 2023, Dr. Yoshimoto was recruited to the Center for Immunobiology in the Department of Investigative Medicine at WMed, bringing her extensive expertise and securing her second R01 along with a new R21 grant. Dr. Yoshimoto was promoted to full professor in July 2024. Dr. Yoshimoto aims to enhance research activities within the institution through collaborations with other research laboratories.
Research Focus
Our research is dedicated to exploring how blood cells develop in mouse embryos, particularly focusing on the emergence of hematopoietic stem cells (HSCs) and progenitor cells from specialized endothelial cells known as hemogenic endothelial cells (HECs). In the early stages of mouse embryo development, the initial blood cells originate in the extra-embryonic yolk sac before the appearance of HSCs. It has been shown that multiple waves of blood formation occurring in various tissues, including the yolk sac, dorsal aorta, and placenta. Notably, our investigations revealed that the first B- and T-lymphoid progenitors also originate from endothelial cells in both the yolk sac and aortic regions independently of HSCs. These HSC-independent B-progenitors tend to mature into innate-like B-1 cells, while HSC-independent T cells demonstrate the potential to mature into various types of T cells. This paradigm-shifting discovery challenges the stem cell dogma that all blood cells stem solely from HSCs. Through the use of lineage tracing mouse models and transplantation assays that label HECs or HSCs, we have demonstrated the enduring presence of HEC-derived (fetal-derived) B- and T-cells into adulthood. Consequently, our focus has shifted to exploring the potential functional differences between HSC-independent and HSC-derived B- and T-cells.
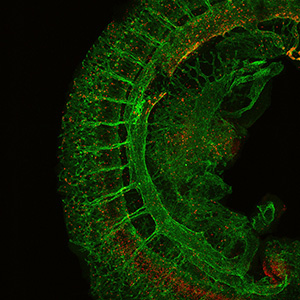
Our research has shown that a significant proportion of B-1 cells originate from HECs, independently of HSCs. B-1 cells play a crucial role by secreting natural IgM antibodies, which bind to oxidized-LDL found in apoptotic cells and atherosclerotic regions. This natural IgM is pivotal in clearing cell debris, curbing chronic inflammation, and preventing atherosclerosis. Our exploration of B-1 cells extends to their potential in alleviating age-related chronic inflammation (Inflammaging) and reducing atherosclerotic regions. Our prior research demonstrated that B cell progenitors derived from mouse ESCs successfully mature into B-1a cells when transplanted into immunodeficient mice. We aim to further investigate if these mouse ESC-derived B-1 cells can effectively impede atherosclerosis development in mouse models.
Additionally, our lineage tracing studies unveiled that intestinal IgA-secreting cells originate during fetal development. Our current R21 project aims to examine the specific roles played by IgA-secreting cells originating from different sources (fetal or HSC-derived) in addressing intestinal infectious conditions.
Moreover, our ongoing research delves into generating functional B-cells and HSPCs from human inducible pluripotent stem cells (iPSCs). This project holds promise not only in comprehending the development of hematopoietic cells in human embryos but also in pioneering immune cell therapy using innate lymphoid cells derived from human iPSCs.
Current Projects
- Determine the hematopoietic lineage trees from endothelial cells in the embryo to adult mice.
- Determine the possible different functions between fetal-derived and HSC-derived B cells.
- Identifying the earliest innate lymphoid progenitors that seed the lamina propria of the intestine.
- Determine the preventive role of B-1 cells against atherosclerosis
- Examining the hematopoietic niche in the fetal liver in mice and humans.
- Inducing functional B cells from mouse and human pluripotent stem cells.
Lab Personnel
 |
|
 . Shun Yonehara . Shun YoneharaResearch Assistant shun.yonehara@wmed.edu |
Recent Publications
Our laboratory group is committed to pursuing inquiry, disseminating knowledge, and fostering critical thinking that encourages lifelong learning. Take a look at a comprehensive listing of Dr. Yoshimoto's most recent publications.



 Hitomi Ura, MD
Hitomi Ura, MD